|
Politics Forum
|
List All Forums | About |
|
|
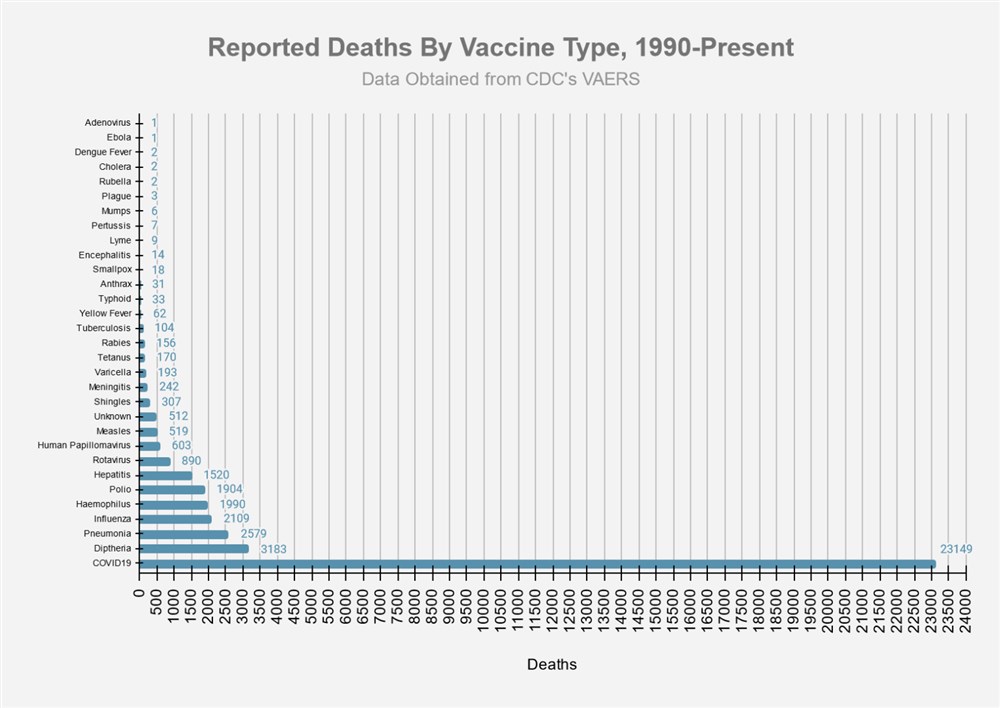
Photo From: Tom Hemmick - Album: No Album
Description: Chart shows how deaths from vaccines for various diseases compare to other diseases. The Covid vaccination deaths are hugely ahead of all other diseases. Updated 1/28/2022 Click Here Uploaded: 2/7/2022 by Tom Hemmick
See what is being said about this image in the messages area.
Comments on this Image: You must be registered user and logged in to comment.
Login or Register and you will be returned to this image page.
Comments on this Image: You must be registered user and logged in to comment.
Login or Register and you will be returned to this image page.
|
(2/11/2022) Jim Matthews wrote:
1.The link you posted is that of a private individual not related in any way to VAERS. 2. Anyone can submit reports to VAERS. The data is not validated. 3. The VAERS website is: https://vaers.hhs.gov/ 4. VAERS DISCLAIMER: VAERS accepts reports of adverse events and reactions that occur following vaccination. Healthcare providers, vaccine manufacturers, and the public can submit reports to the system. While very important in monitoring vaccine safety, VAERS reports alone cannot be used to determine if a vaccine caused or contributed to an adverse event or illness. The reports may contain information that is incomplete, inaccurate, coincidental, or unverifiable. In large part, reports to VAERS are voluntary, which means they are subject to biases. This creates specific limitations on how the data can be used scientifically. Data from VAERS reports should always be interpreted with these limitations in mind. The strengths of VAERS are that it is national in scope and can quickly provide an early warning of a safety problem with a vaccine. As part of CDC and FDA’s multi-system approach to post-licensure vaccine safety monitoring, VAERS is designed to rapidly detect unusual or unexpected patterns of adverse events, also known as “safety signals.” If a safety signal is found in VAERS, further studies can be done in safety systems such as the CDC’s Vaccine Safety Datalink (VSD) or the Clinical Immunization Safety Assessment (CISA) project. These systems do not have the same scientific limitations as VAERS, and can better assess health risks and possible connections between adverse events and a vaccine. Key considerations and limitations of VAERS data: Vaccine providers are encouraged to report any clinically significant health problem following vaccination to VAERS, whether or not they believe the vaccine was the cause. Reports may include incomplete, inaccurate, coincidental and unverified information. The number of reports alone cannot be interpreted or used to reach conclusions about the existence, severity, frequency, or rates of problems associated with vaccines. VAERS data is limited to vaccine adverse event reports received between 1990 and the most recent date for which data are available. VAERS data do not represent all known safety information for a vaccine and should be interpreted in the context of other scientific information. VAERS data available to the public include only the initial report data to VAERS. Updated data which contains data from medical records and corrections reported during follow up are used by the government for analysis. However, for numerous reasons including data consistency, these amended data are not available to the public. |
